
Stark Portable Cov-2 Antigen Lab
The revolution
- NON INVASIVE
- PATIENT SIDE
- HIGH SENSIBILITY
- RESULT IN 30 MINUTES
- EARLY DIAGNOSIS
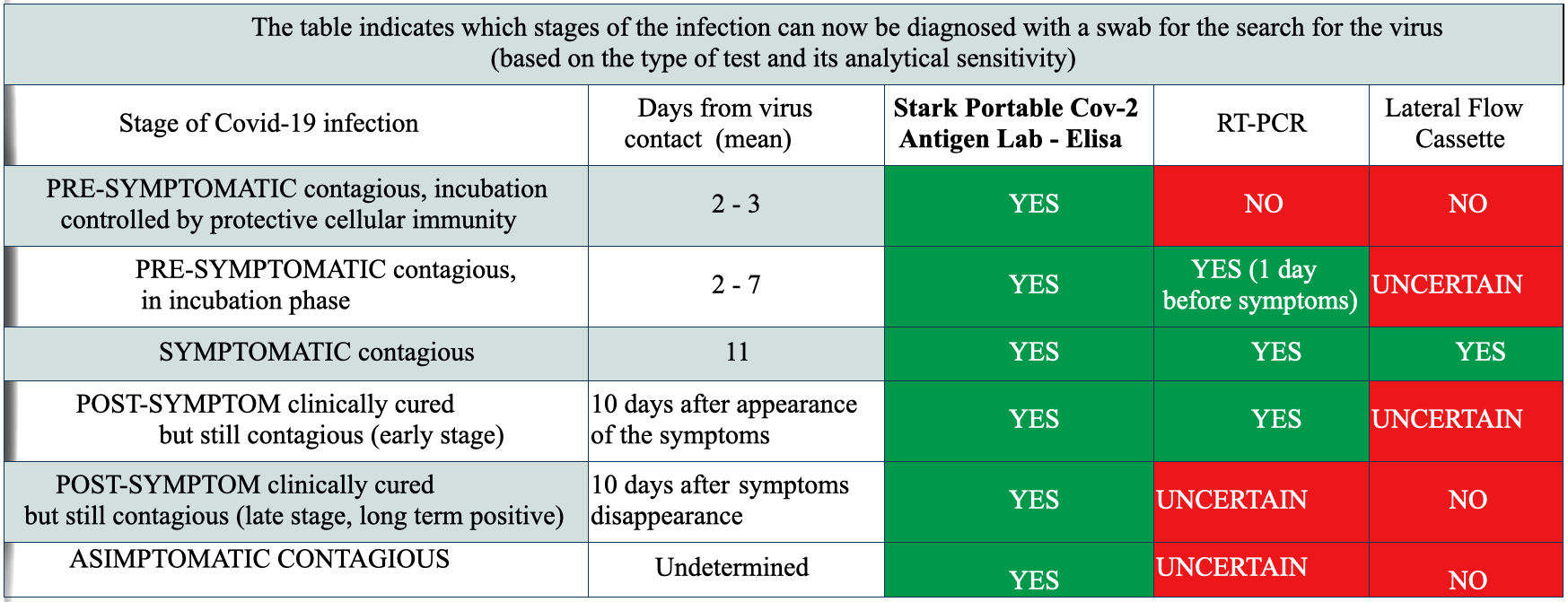
The “Stark Covid-19 portable antigen Lab” test is a highly sensitive portable laboratory for the rapid diagnosis of Covid-19. This method uses the ELISA technique for the detection of Coronavirus N and S antigens in cyto-salivary samples taken in the patient’s mouth with a non-invasive cytological brush. The test takes less than 30 minutes and a single operator can develop up to 20 at the same time. The search for the Nucleocapsid antigen makes it effective against all Spike variants of the coronavirus.
The cyto-salivary brush collects, in addition to a sample of saliva, also thousands of cells that release viral antigens in the lysis station of the test, which are concentrated and collected on a detector palette by specific antibodies. The palette, passing through a sequence of development stations, shows, in case of positivity, a pink colored band obtained through a chemo-colorimetric reaction. The ELISA technique has been a gold standard of diagnostics since 1972, it differs from other diagnostic methods commonly used for Covid-19 and is characterized by a high diagnostic accuracy in terms of specificity and sensitivity that cannot be matched by other methods. In particular, this test has a wider diagnostic window than the others and is able to find the virus even in the pre-symptomatic period, when the other tests are still ineffective. This feature makes it particularly suitable for hospital rule-in because it reduces the risk of admitting an incubating patient not yet positive to other tests such as RT-PCR or LFC antigen in a clean ward. Even in hospital Rule-out, this test can be useful in accurately determining the disappearance of viral antigens when broken RNA chains of no longer active viruses are still visible on RT-PCR for weeks even if the patient has already defeated the infection. In addition, the “Stark Covid-19 portable antigen Lab” test is rapid and can therefore reduce the wait for patients entering the emergency room, also freeing staff engaged in managing the gray area and spaces in which patients are kept under observation. The non-invasive sampling makes the “Stark Covid-19 portable antigen Lab” test particularly suitable for pediatric hospitals, children, the disabled, the elderly and schools. The early diagnosis that can be done with this test also makes it useful in screening sportsmen before retreats or competitions, students before exams, workers in crowded companies, military rallies. It can also be used to travel safely by plane, ship and for cruises, tourist stays within villages or traveling groups. The high diagnostic accuracy of the “Stark Covid-19 portable antigen Lab” test makes it particularly useful for monitoring the cohabitants of infected patients in families, communities and nursing homes.
The ultra-sensitive portable laboratory
Stark Portable Cov-2 Antigen Lab is the new patented test for the early diagnosis of coronavirus infection.
A real portable laboratory in kit.
CE certified and registered at the Italian Ministry of Health.
The result is obtained in 30 minutes, patient side. This newly developed antigen test detects the viral protein fraction Nucleocapsid. It allows to identify the incubation phase, the early phases of the infection even with a low antibody response and symptoms not yet evident, accurately identifies the end of the quarantine period and identifies the variants of Covid-19 with Spike mutation.
Efficacy of COVID-19 Swab Tests
Non-invasive
lingual swab
The type of cytological brush sampling that is used for the test Stark Portable Cov-2 Antigen Lab collects cells from the posterior part of the tongue where the virus is present in greater quantities.
It therefore allows to increase the concentration of viral antigens compared to saliva samples alone or mucus, in which the virus is diluted e therefore more difficult to find
References
Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [CrossRef] [PubMed]
World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. 2020. Available online: https://covid19.who.int (accessed on 22 October 2020).
WHO. Coronavirus Disease (COVID-2019) Situation Report–51. 2020. Available online: https://www.who.int/docs/default- source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10%2011%20March (accessed on 24 November 2020).
WHO. Laboratory Diagnostics for Novel Coronavirus. 2020. Available online: https://www.who.int/emergencies/diseases/ novel-coronavirus-2019/technical-guidance/laboratory (accessed on 27 January 2021).
Nishiura, H.; Kobayashi, T.; Suzuki, A.; Jung, S.M.; Hayashi, K.; Kinoshita, R. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int. J. Infect. Dis. 2020, 94, 154–155. [CrossRef] [PubMed]
Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. EuroSurveillance 2020, 25, 2000180. [CrossRef] [PubMed]
7. European Centre for Disease Prevention and Control, Disease background of COVID-19 (https://www.ecdc.europa.eu/en/2019-ncov-background-disease)
8. WHO Director-General’s opening remarks at the media briefing on COVID-19 – 11 March 2020
(https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020)
9. Centers for Disease Control and Prevention, Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19
(https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html)
10. Mina M.J., Parker R., Larremore D. B., “Rethinking Covid-19 Test Sensitivity — A Strategy for Containment”, NEJM, 2020, doi: 10.1056/NEJMp2025631 https://www.nejm.org/doi/full/10.1056/NEJMp2025631
11. https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostictests-for-covid-19